 When we heard about the world’s first synthetic lifeform, we realized we needed a science expert to help us explore and share the real significance of the news. So we’re very pleased to introduce our first guest blogger, Alexandra Ruaux (wife of regular contributor Alexander Bowyer). Alexandra has a PhD in Biochemistry from the University of Southampton, UK and is currently a postdoctoral researcher at the Biotechnology Research Institute in Montréal, where she spends most of her days growing and isolating bacterial proteins to determine their molecular structure. She has published several journal papers and contributed structures to the Protein Data Bank.
When we heard about the world’s first synthetic lifeform, we realized we needed a science expert to help us explore and share the real significance of the news. So we’re very pleased to introduce our first guest blogger, Alexandra Ruaux (wife of regular contributor Alexander Bowyer). Alexandra has a PhD in Biochemistry from the University of Southampton, UK and is currently a postdoctoral researcher at the Biotechnology Research Institute in Montréal, where she spends most of her days growing and isolating bacterial proteins to determine their molecular structure. She has published several journal papers and contributed structures to the Protein Data Bank.
Synthia is the first living organism on the planet to have a computer for a parent. From just four bottles of chemicals (the basic components of DNA; Adenine, Cytosine, Guanine and Thymine) Dr. Craig Venter and his research group at the J. Craig Venter Institute synthesised the entire genome of a bacterial cell. They used a known genetic code as a recipe and transplanted it into a different cell, effectively causing it to ‘change’ species. What does this mean for mankind? Are we about to have the magic of life reduced to just 4 chemicals? Will terrorists be able to synthesise terrible bio-weapons? Can we now design cells that do whatever we want?
For now the answer to all of those questions is no, but Venter’s work is important because it brings the technology, and the discussion of its implications and ethics, to people’s attention as something more than science-fiction. What has been accomplished by this group, and reported in Science, is a milestone in biotechnological advances and will no doubt provide a stepping stone to many major achievements in the future, good and bad.
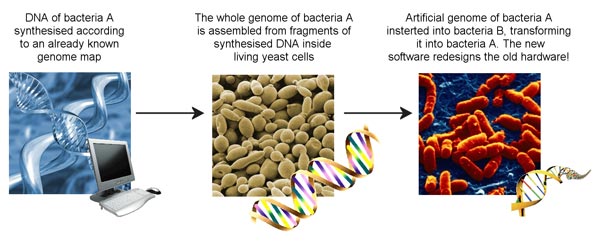
What this group of scientists have achieved is to synthesise overlapping fragments of DNA from the genome of one type of bacterial cell (Mycoplasma mycoides), piece them together inside yeast cells and then insert this whole genome into a bacterial cell of a different species (Mycoplasma capricolum). The result was that after many generations of replication, the daughter cells of the M.capricolum no longer contained (or consisted of) any molecules that were encoded by its own genome. Instead, everything had been made by following the instructions on the transplanted synthetic genome of M. mycoides. In nature, both of these bacteria infect goats and cause diseases of the lung, but there are subtle differences between them, particularly at the molecular level and it is here that the reprogramming of the cell can be observed. The new software in the cell was able to build its own hardware.
This convergence of two significant biological engineering feats, synthetically writing a large genomic recipe and devising a method for transplanting it into a recipient cell and ‘boot’ from it, is a very involved process. Dr. Craig Venter, the head of the group, clearly explains in a TED talk how they did it. Each of these advances is an amazing and useful biological tool, but combined, they provide a means by which scientists can try to piece together the basics of life.
For years we’ve been able to alter, delete or add DNA to a bacterial genome, but now we can systematically strip down the genome to the essential genes required for life and replication. Cells are very complex systems and each gene can create several components, all of which work together in a wonderfully intricate manner to form interconnected networks and pathways. The ability to study a simplified, but ‘real’ system, such as is provided by this synthetic cell, is invaluable in understanding the complex and multifaceted process that take place within all cells.
But the implications of the ability to create a synthetic cell go far beyond merely understanding how a cell works. The recipient (M.capricolum) and donor (M. mycoides) cells in this experiment were chosen because they are genetically quite similar, therefore increasing the chances of success. The hope is that a ‘universal recipient cell’ can be engineered into which we can begin to write any of our own DNA recipes, without having to worry about compatibility, a bit like a universal power adapter. By understanding how the systems and their components function within a cell we will be able to ‘engineer’ gene products that could, for example, degrade crude oil and be used to clean up in situations such as the Louisiana oil spill. Or we might tackle global warming by engineering cells that can use carbon dioxide as a starting material to create a fuel.
We shouldn’t get too ahead of ourselves, though. Synthia is really just a proof of concept and we’re many years off seeing this technology become widespread. Synthetic life has now been created, at a cost of over $40 million and 15 years of dedicated research and brute force chemical synthesis. Hopefully it will follow the example of sequencing the human genome and we will see costs decrease, where what once took 13 years to complete can now be done in less than a month and at a fraction of the cost. Don’t worry, we’re not going to see terrorist groups trying to use this method anytime soon.
We don’t yet know how transferable this technology is. Venter’s group took 2 organisms with 10% difference in their genomes (about the same as between humans and mice) and managed to overcome many difficulties to achieve the genomic transplantation. The development of a universal recipient cell, now one of the group’s goals, would be an important achievement if this technology is to ever go mainstream. There’s also no danger of life being reduced to a bag of chemicals just yet. After all, even though machines built the starting DNA fragments, live yeast cells were required to act as nano-factories to assemble them correctly and the genome was transplanted into whole recipient cells.
We’re still a very long way off being able to truly synthesise life without relying on Mother Nature to provide components. This biotechnological milestone will affect our lives in the future, but for now it’s a step forward into new and interesting areas of science.
Image credits:
The green man image is from a different experiment which created a human-shape from human cancer cells at the University of Tokyo in 2009.
The flowchart was made by Alexandra Bowyer.
Images of the synthetic cell can be found here.






 @
@
 Tags:
Tags: 


 Like all images on the site, the topic icons are based on images used under Creative Commons or in the public domain. Originals can be found from the following links. Thanks to
Like all images on the site, the topic icons are based on images used under Creative Commons or in the public domain. Originals can be found from the following links. Thanks to
[…] Our guest post by Alexandra Bowyer, Is this the beginning of synthetic life? […]